Loading... Please wait...
Loading... Please wait...- Home
- Anti Estrogens
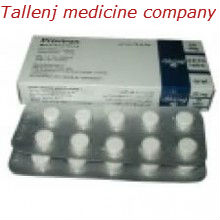
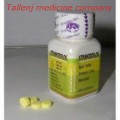
- Proviron 25mg x 20 tabs
Product Description
Type a description for this product herROVIRON TABLETS SCHEDULING STATUS: S5 PROPRIETARY NAME (and dosage form): PROVIRON TABLETS Oral androgen for the treatment of male patients COMPOSITION 1 tablet contains mesterolone (17beta-hydroxy-1alpha-methyl-5alpha-androstan-3-one) 25 mg and the preservatives methylparaben (0,02%) and propylparaben (0,01%). PHARMACOLOGICAL CLASSIFICATION A. 21.7 Male sex hormones. PHARMACOLOGICAL ACTION Proviron balances a deficiency of androgen formation which begins to fall gradually with increasing age. Therefore, Proviron is suitable for treatment of all conditions caused by deficient endogenous androgen formation. In the recommended therapeutic dosage, Proviron will not impair spermatogenesis. Proviron is especially well tolerated by the liver. INDICATIONS • Declining physical activity and mental alertness in middle- and old-aged men Reduced efficiency, easy fatigability, lack of concentration, weak memory, disturbances of libido and potency, irritability, disturbances of sleep, depressive moods, and general vegetative complaints are often attributed to androgen-deficiency. These complaints can be overcome or improved by the use of Proviron tablets. • Potency disturbances Proviron overcomes potency disturbances due to androgen-deficiency. It may also be of use as supplementary therapy in cases of diminished potency where androgen-deficiency is not the primary cause. • Hypogonadism Growth, development and function of androgen-dependent target organs are stimulated by Proviron. It promotes development of secondary male sex characteristics in cases of prepuberal hypogonadism. Full clinical and laboratory investigations are necessary in all cases of young patients prior to commencement of treatment. Proviron tablets may also be used as a substitution therapy in cases where a loss of gonadal function has occurred post-puberally. • Infertility Oligozoospermia and deficient Leydig-cell secretion may be the cause of infertility. With Proviron treatment, sperm count can be increased, the quality improved and, furthermore, a higher fructose concentration up to normal values can be achieved thus increasing the chances of procreation. CONTRA-INDICATIONS In patients with carcinoma of the prostate, androgen therapy of any kind, including the use of Proviron, is contra-indicated. DOSAGE AND DIRECTIONS FOR USmbined therapy with gonadotrophic hormone exhibiting FSH activity is recommended for the commencement of treatment (eg 2000 IU serum gonadotrophin im twice weekly up to a total amount of 12 000 IU). If necessary, Proviron treatment is to be repeated after an interval of several weeks. SIDE EFFECTS AND SPECIAL PRECAUTIONS The administration of Proviron is recommended only for male patients. Regular examinations of the prostate should be carried out prophylactically. KNOWN SYMPTOMS OF OVERDOSAGE AND PARTICULARS OF ITS TREATMENT None. IDENTIFICATION Small, white, round tablets with flat sides and bevelled edges. Upper side: impressed with AX in an equilateral hexagon. Lower side: scored. PRESENTATION 20 and 100 tablets. STORAGE INSTRUCTIONS Store below 30°C. For shelf-life, refer to the imprint on the pack. Keep out of reach of childrene...